40 drug warning labels examples
FDA Label Search - Food and Drug Administration The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor ... Academic Journals | American Marketing Association Journal of Marketing (JM) develops and disseminates knowledge about real-world marketing questions useful to scholars, educators, managers, policy makers, consumers, and other societal stakeholders around the world.It is the premier outlet for substantive marketing scholarship. Since its founding in 1936, JM has played a significant role in shaping the content and boundaries of …
Prescription Label Design: Why It Matters and Effective Examples - Etactics Consumer Reports staffers wanted to compare drug labels, warnings, and information. They filled prescriptions for the drug warfarin at five pharmacies ... Details can be misleading if the phrasing is unclear. For example, the Costco label that I mentioned earlier did include the necessary warning but the statement made it unclear about what was ...

Drug warning labels examples
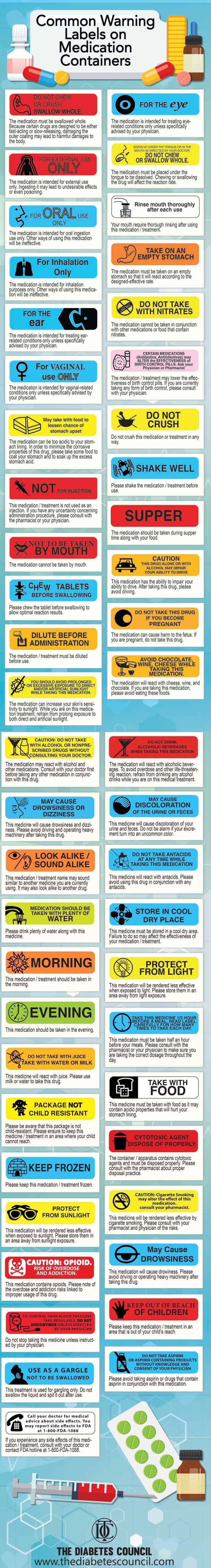
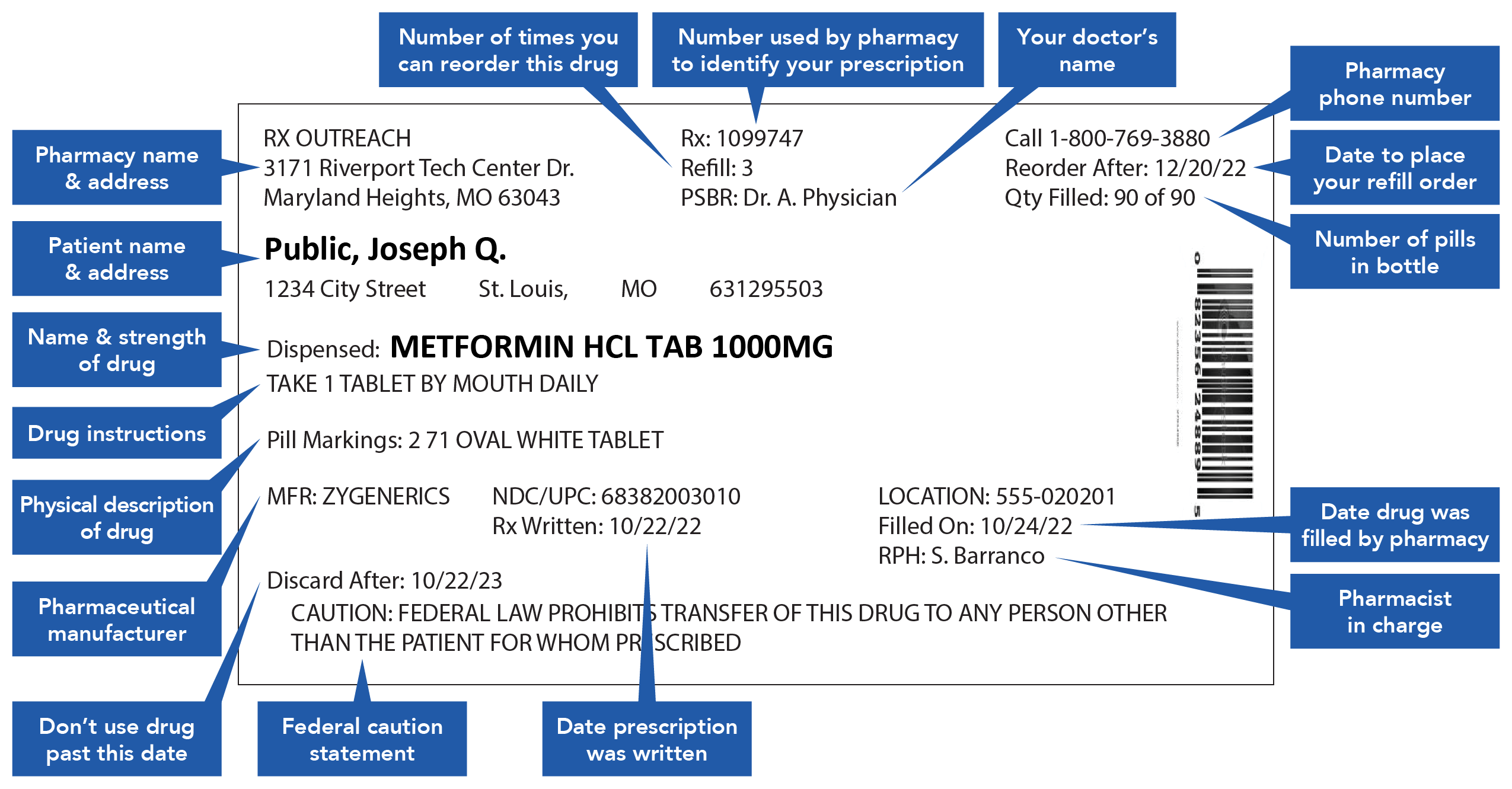
What Information Should Be on Drug Labels? - MedicineNet Before any drug can be legally sold, it must be assigned a Drug Identification Number (DIN). The FDA recommends manufacturers to provide data demonstrating product safety, effectiveness in meeting label claims, potency, and purity. When the manufacturer meets these requirements, the FDA assigns the drug a registration number. A Warning About Warning Labels - U.S. Pharmacist "Do not chew or crush, swallow whole" was misinterpreted as "do not swallow whole" or "chew it up so it will dissolve," suggesting patients had read some, but not all, of the words on the label. Icons or Symbols: Adults with low literacy may rely more heavily on symbols to interpret the meaning of labels. Labeling guidelines for sample prescription drugs | Mass.gov Practitioners must label all sample medications dispensed to patients, including those provided as part of an indigent patient drug program (see M.G.L. c. 94C §22 and 105 CMR 700.010). Labels must contain the information described below; however, the method of labeling the medications may vary. For example, sample medications may be placed in ...
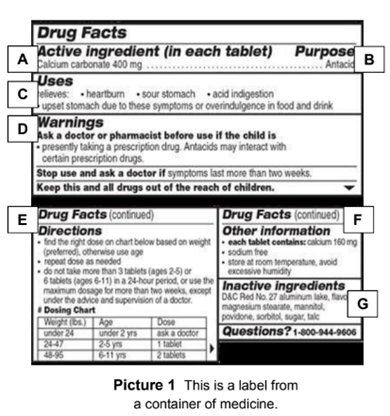
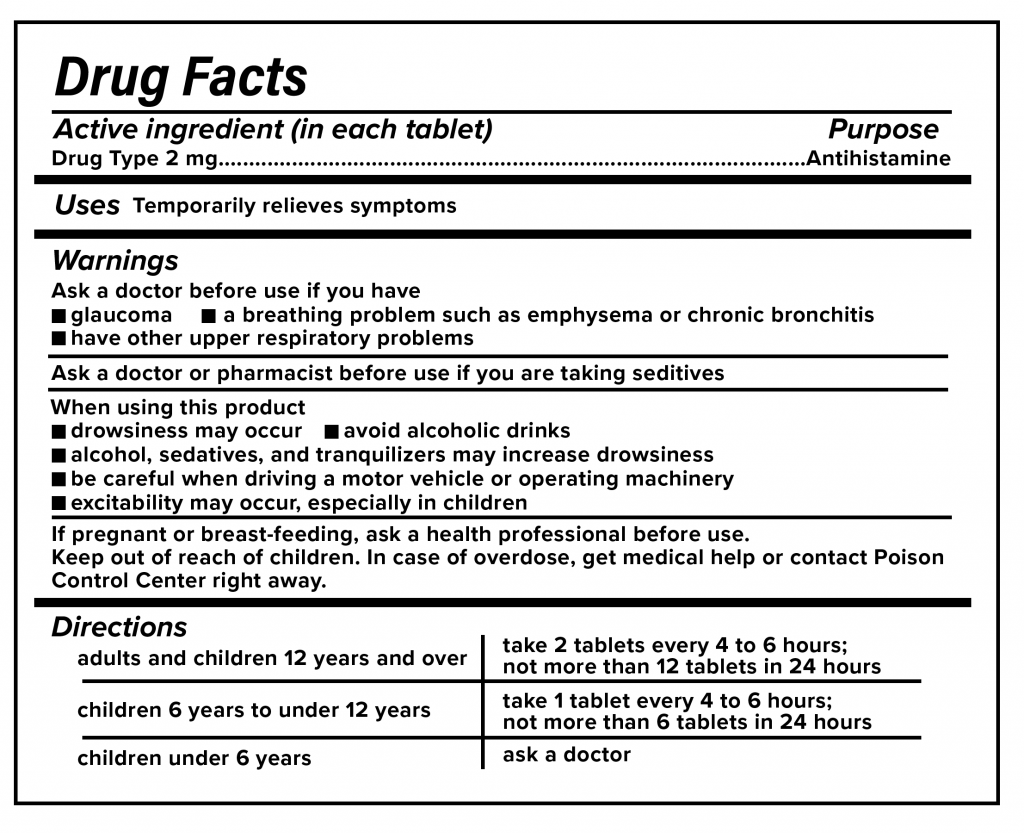
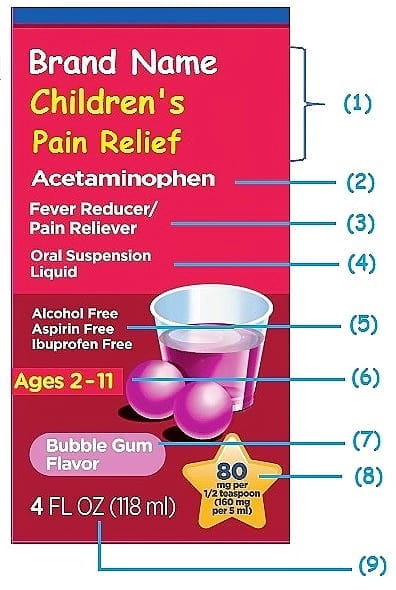
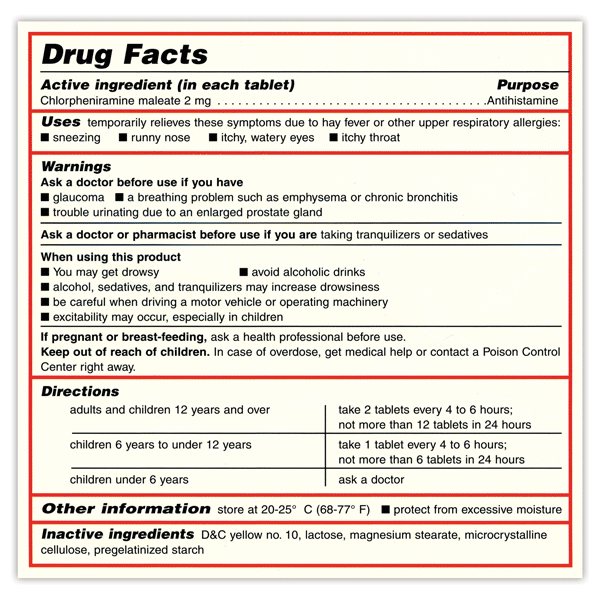
Drug warning labels examples. OTC Drug Facts Label | FDA Along with the standardized format, the label uses plain-speaking terms to describe the facts about each OTC drug. For example, "uses" replaces "indications," while other technical words like... Drug Interactions: What You Should Know | FDA Over-the-counter (OTC) drug labels contain information about ingredients, uses, warnings and directions that is important to read and understand. The label also includes important information ... Labeling Information | Drug Products | FDA For more information on labeling, including Physician Labeling Rule (PLR) requirements, guidances, presentations, sample templates and format tools, and established pharmacologic class (EPC ... Sample Drug Facts Label | FDA Sample Drug Facts Label. Use Caution When Giving Cough and Cold Products to Kids.
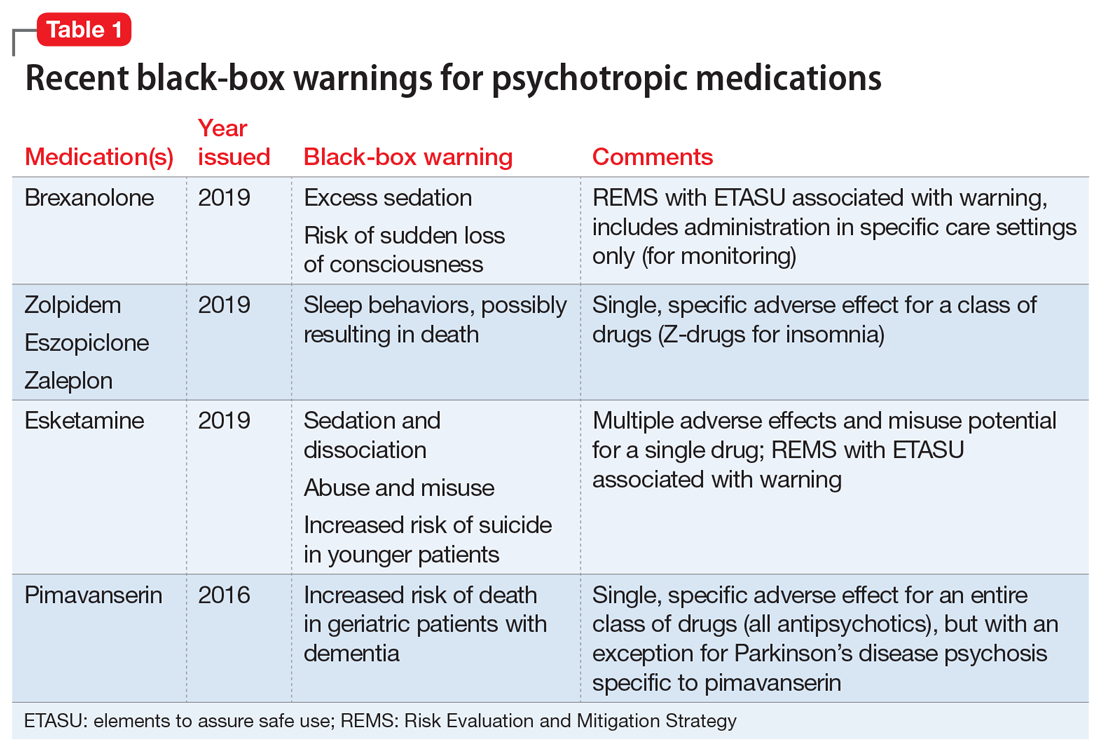
FDA's Labeling Resources for Human Prescription Drugs | FDA Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective use of the drug; and (2) includes the Prescribing Information,... Pharmacy, Medication Warning Labels & Stickers | PDC Medication Warning Labels, Medication Warning Labels, Items 1 - 32 of 262, Sort By, Compare, Warning Label "CAUTION: OPIOID Risk of Overdose and Addiction" 1-9/16" x 3/8", Red with White Text, Permanent, 500 per Roll, 2 Rolls per Box, 1-1197, $6.15, Add to Cart, Compare, The Over-the-Counter Medicine Label: Take a Look | FDA All nonprescription, over-the-counter (OTC) medicine labels have detailed usage and warning information so consumers can properly choose and use the products. Below is an example of what the OTC... 10 Medications with Black Box Warnings You Must Know About Febuxostat (Uloric). Indicated for the chronic management of hyperuricemia in adults with gout, Febuxostat received a black box warning in February 2019, alerting prescribers and patients of the higher rate of CV mortality associated with the drug's use compared with allopurinol, its most common competitor. Ticagrelor (Brilinta).
Example drug labeling API queries This query searches for labels with a Boxed Warning, and returns one result. The _exists_ search modifier lets you search for records that contain a specific field, ... Example query. Count of drug labeling, by product type. There are more labeling records for over-the-counter (OTC) drugs than prescription drugs. ... FDALabel: Full-Text Search of Drug Product Labeling | FDA For example, recent publications describe how information in drug labeling can be used to aid and facilitate drug repurposing 4 as well as applications in precision medicine, drug safety, and ... FDA Black Box Warnings of 8 Very Common Drugs: Read Your Labels Avelox tendon ruptures can be very severe, and lead to life-long disability. 5. Black Box Warning for Synthroid (Levothyroxine ) (for Hypothyroidism) Synthroid (levothyroxine) is the number one brand-name drug in terms of number of prescriptions written by physicians - over 23 million scrips/month. 13+ Verbal Warning Follow-up Letter Templates | Free Samples, Examples … Functions of Verbal Warning Letters. Part of knowing how to properly use the tools you have is understanding what they can do for you. In this case, being aware of the functions of a written warning letter should make you all the more adept at using them yourself. Of course, you should be aware of what functions verbal warning letters do have in order to find out exactly how you …
Implementation Guidelines for Alcohol and Drug Regulations DOT's final rule, 49 CFR part 40, "Procedures for Transportation Workplace Drug and Alcohol Testing Programs," took effect August 1, 2000. ... Examples written instructions for drivers and collection site personnel are provided in the appendix at the end of this chapter. ... The MRO must give this warning to the driver before obtaining any ...
Boxed warning - Wikipedia In the United States, a boxed warning (sometimes "black box warning", colloquially) is a type of warning that appears on the package insert for certain prescription drugs, so called because the U.S. Food and Drug Administration specifies that it is formatted with a 'box' or border around the text. The FDA can require a pharmaceutical company to place a boxed warning on the …
Understanding Food Labels | The Nutrition Source | Harvard … All FOP labels in the U.S. are voluntary, which allows food manufacturers to highlight or hide the nutrition information they choose to help promote or preserve sales. If warning labels became mandatory, as public health advocates propose, the pressure on manufacturers would increase to change certain products to improve their nutritional quality.
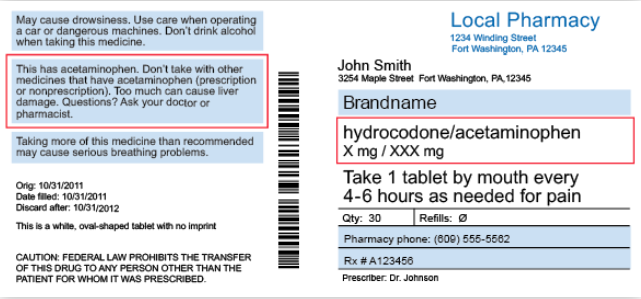
Custom Labels That Support Good Health Outcomes - Drug Package Common examples of auxiliary label warnings and instructions include: "May cause drowsiness", "Keep in Refrigerator", "Shake well before using", "Do not crush / swallow whole", Pharmacy auxiliary labels that contain common messages can be purchased as readymade products that the supplier doesn't alter before sending the order.
Cautionary and advisory labels | About | BNF | NICE To be used with label 25 on preparations coated to resist gastric acid (e.g. enteric-coated tablets). This is to avoid the possibility of premature dissolution of the coating in the presence of an alkaline pH. Label 5 also applies to drugs such as gabapentin where the absorption is significantly affected by antacids.Pharmacists will be aware (from a knowledge of physiology) that the usual time ...
Mercola.com, LLC - 607133 - 02/18/2021 | FDA - U.S. Food and Drug ... Mar 04, 2021 · WARNING LETTER. Date: February 18, 2021 ... Some examples of the claims on your websites that establish the intended use of your products, based on statements about the purported effects of their ...
Perceptions of prescription warning labels within an underserved ... Prescription warning labels are small colored stickers placed adjacent to the drug label on a prescription bottle that provides important cautionary information concerning the safe administration of a medicine. For example, "take with food" or "limit time in sunlight when taking this medication".
50 Common Warning Labels On Medication Containers Top 50 Common Warning Labels and Their Meanings, The medication must be swallowed whole. Because certain drugs are designed to be either fast-acting or slow-releasing, damaging the outer coating may lead to harmful damages to the body. The medication is intended for external use only. Ingesting it may lead to undesirable effects or even poisoning.
6 Medicines That Have The Freakiest Warning Labels Why It's On This List: "SIDE EFFECTS that may occur while taking this medicine include blurred vision, constipation, decreased sexual desire or ability, diarrhea, dizziness, drowsiness, dry mouth, headache, increased sweating, loss of appetite, muscle aches, nausea, sore throat, tiredness, trouble sleeping, or vomiting.
Examples of Metaphors in Literature - ProWritingAid Jan 14, 2022 · Examples of Metaphors. Now that we know what a metaphor is, let’s take a closer look at some examples of metaphors at work in the real world. Metaphor Examples from Literature “The sun was a toddler insistently refusing to go to bed: It was past eight thirty and still light.”—Fault in Our Stars, John Green
Common Grapefruit Juice Drug Interactions - Drugs.com May 18, 2022 · While grapefruit is a nutritious fruit, many patients are concerned about the potential for drug interactions with grapefruit juice. Maybe you've receive a medication prescription container with an affixed warning label that recommends you avoid grapefruit or grapefruit juice while taking the medication.
Warning label - Wikipedia The examples and perspective in this article may not represent a worldwide view of the subject. ... Drug, and Cosmetic Act of 1938. ... Warning labels have been produced for different items. In some cases, rumors have developed of labels warning against some very strange occurrences, such as the legendary microwave warning that allegedly states ...
Labeling guidelines for sample prescription drugs | Mass.gov Practitioners must label all sample medications dispensed to patients, including those provided as part of an indigent patient drug program (see M.G.L. c. 94C §22 and 105 CMR 700.010). Labels must contain the information described below; however, the method of labeling the medications may vary. For example, sample medications may be placed in ...
A Warning About Warning Labels - U.S. Pharmacist "Do not chew or crush, swallow whole" was misinterpreted as "do not swallow whole" or "chew it up so it will dissolve," suggesting patients had read some, but not all, of the words on the label. Icons or Symbols: Adults with low literacy may rely more heavily on symbols to interpret the meaning of labels.
What Information Should Be on Drug Labels? - MedicineNet Before any drug can be legally sold, it must be assigned a Drug Identification Number (DIN). The FDA recommends manufacturers to provide data demonstrating product safety, effectiveness in meeting label claims, potency, and purity. When the manufacturer meets these requirements, the FDA assigns the drug a registration number.















![PDF] Improving prescription drug warnings to promote patient ...](https://d3i71xaburhd42.cloudfront.net/e087ba4ebaa8ed043bf22bd2ab63292d6a05b9d4/3-Figure2-1.png)










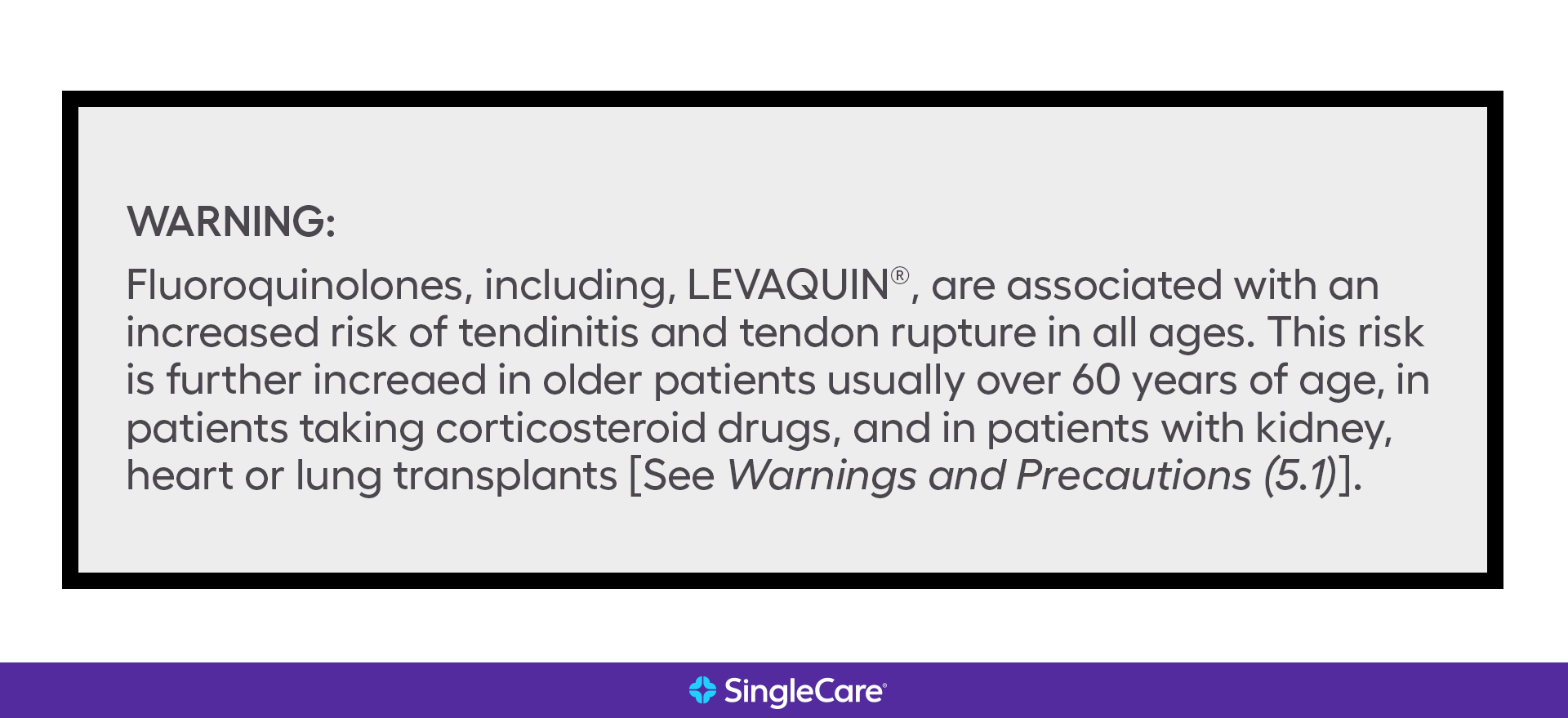
Post a Comment for "40 drug warning labels examples"